ISRA FACTSHEETS
ISRA FACTSHEETS
SOUTH AMERICAN INLAND WATERS
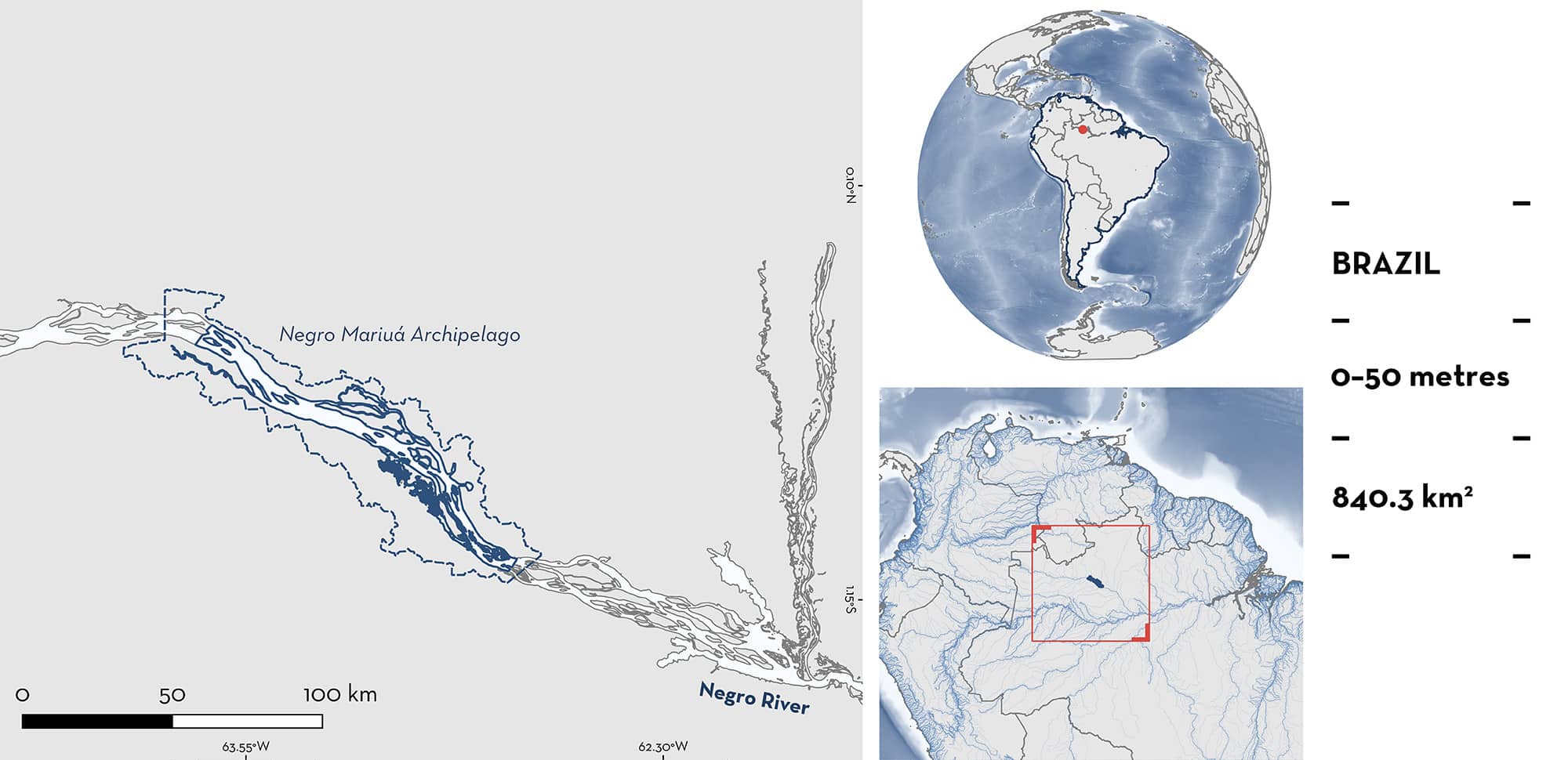
Negro Mariuá Archipelago
Summary
Negro Mariuá Archipelago is located in the middle section of the Rio Negro in the state of Amazonas, Brazil. This area is characterised by islands with sandy beach margins, litter-rich banks, and permanently flooded forest. The area overlaps with the Rio Negro Ramsar Site. Within this area there are: threatened species (e.g., Discus Stingray Paratrygon aiereba); range-restricted species (e.g., Wallace’s Freshwater Stingray Potamotrygon wallacei); reproductive areas (e.g., Schroeder’s Freshwater Stingray Potamotrygon schroederi); and the area sustains a high diversity of rays (3 species).
Download factsheet
Negro Mariuá Archipelago
DESCRIPTION OF HABITAT
Negro Mariuá Archipelago is located in the middle section of the Rio Negro in the municipality of Barcelos, state of Amazonas, Brazil. It is recognised as one of the largest river archipelagos in the world, with over 1,400 islands spread across a stretch of ~140 km (Latrubesse & Stevaux 2015). This area is within the Amazon Basin.
The Negro River originates in Colombia and flows into Brazil’s Amazonas state, passing through São Gabriel da Cachoeira. As it continues eastward, the river forms the vast Mariuá Archipelago after the town of Santa Isabel do Rio Negro, creating a braided middle stretch that extends southeast (Latrubesse 2008). The Negro River is distinguished by its unique physicochemical properties and is the largest blackwater river in the world (Latrubesse et al. 2005).
The Mariuá Archipelago is characterised by islands with sandy beach margins and litter-rich banks located within the main river channel. Here, deeper zones alternate with sandbanks (Araújo 1998), alongside streams and permanently flooded forest (igapó; Araújo et al. submitted c) areas that border the main channel. This area is a unique freshwater habitat in South America, shaped by the blackwater river—a result of organic matter decomposition from surrounding forests, making the water acidic and nutrient-poor. These characteristics distinguish it from other major Amazonian rivers (Duncan & Fernandes 2010).
The Negro River experiences seasonal water level fluctuations, with peak flooding (high water) from May–July, low water levels in August–September, minimum flooding (dry water) from October–December, and rising water from January–April (Araújo 2011). Its extreme seasonal variability, marked by dramatic flooding cycles, and the continuous interaction between land and water due to permanently flooded forests, further distinguish this ecosystem.
This area overlaps with the Rio Negro Ramsar Site (Ramsar 2025).
This Important Shark and Ray Area is benthic and is delineated from surface waters (0 m) to 50 m based on the bathymetry of the area.
CRITERION A
VULNERABILITY
Three Qualifying Species considered threatened with extinction according to the IUCN Red List of Threatened Species regularly occur in the area. These are the Critically Endangered Discus Stingray (Araújo et al. submitted b), the Endangered Wallace’s Freshwater Stingray (Araújo et al. submitted c), and the Vulnerable Schroeder’s Freshwater Stingray (Araújo et al. submitted a).
CRITERION B
RANGE RESTRICTED
This area holds the regular presence of Schroeder’s Freshwater Stingray and Wallace’s Freshwater Stingray as resident range-restricted species. This area, characterised by the unique environmental conditions of the Mariuá Archipelago and its permanently flooded forests, provides a critical habitat that supports multiple life-stages of Schroeder’s Freshwater Stingrays and Wallace’s Freshwater Stingrays, offering essential conditions for reproduction, juvenile development, and adult survival.
Schroeder’s Freshwater Stingray is primarily found in blackwater and clearwater rivers with strong currents and high-water quality, including oxygen-rich conditions (Duncan & Fernandes 2010; Lasso et al. 2013). It is commonly observed in the shallow waters near the beaches of the Negro River islands, within this area, where the hydrological and environmental conditions closely match its habitat preferences (Duncan & Fernandes 2010, Duncan et al. 2021). Schroeder’s Freshwater Stingrays display ontogenetic and sexual segregation, with adults occurring in the main river channel and neonates and juveniles in shallow sandy banks, where there is high water current flow (Araújo et al. 2005). Expeditions conducted between 2010 and 2015 have confirmed the contemporary presence of this species in the area, with twelve individuals collected (Araújo Alves 2013; Ictiologia Collection – Instituto Nacional de Pesquisas da Amazônia, unpubl data 2015; Neris Machado 2016). Schroeder’s Freshwater Stingray is restricted to the Amazon Basin, mainly in the upper-mid Negro River Basin (Brazil) and in the upper-mid Orinoco River Basin (Venezuela, Colombia) (Araújo et al. submitted a).
The highest known population density of Wallace’s Freshwater Stingray is located within this area, in the Itu-Daraquá-Bafuana River system, and adjacent to the flooded forests, on the left bank of the Negro River, ~100 km upstream from the city of Barcelos, Amazonas (Araújo 1998). Wallace’s Freshwater Stingray prefers leaf-littered substrates in shallow, acidic waters (pH ~4.5) characterised by low oxygen levels and an average temperature of ~25ºC. These conditions are predominantly found in this unique ecosystem. This species exhibits an unusual morphology in its gills (Duncan & Fernandes 2010) and possesses physiological adaptations that enable it to tolerate acidic and highly diluted waters (Wood et al. 2002). Individuals show limited seasonal movements within the habitat, following flood and ebb cycles, when they respectively enter the flooded forest or congregate around islands in the main channels. The species is strictly confined to this specialised habitat (Araújo 1998; Duncan & Fernandes 2010; Carvalho et al. 2016; Duncan et al. 2016) and the longest travelled distance by a tagged male specimen was 15 km (ML Araújo unpubl. data 2016; Carvalho et al. 2016). Several expeditions between 2006–2018 collected 848 Wallace’s Freshwater Stingray (with 438 individuals collected between 2012–2018) in various life-stages, including neonates and young-of-the-year (YOY), pregnant females, and sexually active males (Duncan et al. 2016; de Oliveira et al. 2016, 2021; Marcon et al. 2021; Siqueira‐Souza et al. 2024). This indicates that this species uses this area across life-stages. Wallace’s Freshwater Stingray is endemic to the middle Negro River Basin in Brazil, with its distribution ranging from above the confluence with the Branco River (in Barcelos, Brazil) to below the confluence with the Téa River (in Santa Izabel do Rio Negro, Brazil) (Araújo et al. submitted c).
CRITERION C
SUB-CRITERION C1 – REPRODUCTIVE AREAS
Negro Mariuá Archipelago is an important reproductive area for three ray species.
From May 1996 to October 2008, 227 Discus Stingrays (males = 108, females = 119) were sampled through scientific fishing using hand nets and longlines (Araújo 2011). The specimens were collected in six locations within this area, each representing a variety of habitats such as flooded forest, sandy beaches, or the main river channel. Across 109 sampling days, the species occurred in 44% of the captures (Araújo 2011). Sixty-eight individuals (30%) were below 24.5 cm disc width (DW). Age estimated through marginal increment analysis of central vertebrae revealed that 34 individuals (15%) were neonates or YOY (Araújo 2011). Pregnant females were found in the dry season (n = 2) and at the beginning of the flood season (n = 1). Of the three pregnant females analysed, two had full-term embryos with vestigial yolk sac. The body sizes for these embryos were between 16–17.1 cm DW (Araújo 2011). The smallest individuals observed in the wild were 15.3 cm DW for females and 16.4 cm DW for males (Araújo 2011). The estimated average birth size was 16.3 cm DW. In March 2003, during the flood period, a birthing event was observed (Araújo 2011). The remaining adult females (n = 5) were in reproductive rest and were captured at the beginning of the dry season, and postpartum females were captured during the flood season (Araújo 2011). One postpartum female captured at the beginning of the flood season had scars and abrasions caused by bites from a male. Males with sperm in the seminal fluid were found at the beginning of the dry season and flood season (Araújo 2011). Mating behavior was observed in January–February, and ovulation occurred early in August (Araújo 2011). Gestation lasted 4–6 months with parturition occurring in late January–February (Araújo 2011). In addition, during 10 field expeditions from December 2006 to October 2010, 48 Discus Stingrays were caught through scientific fishing using hand nets. Individuals sampled included nine neonates, 16 YOYs, 20 subadults, and three adults (one male and two females, of which one was pregnant) (de Oliveira et al. 2016). In another study conducted between 2012–2015, 19 Discus Stingrays were sampled in this area through scientific fishing using bottom longlines, gillnets, cast nets, seine nets, and hand nets (Duncan et al. 2021). Individuals had an average body size of 26.4 cm DW (± 0.7 SD) with a range of 18–38 cm DW (Duncan et al. 2021). Therefore, some of these individuals were YOYs or juveniles.
Historical evidence from the mid-1990’s indicates that Schroeder’s Freshwater Stingrays use this area for reproduction. From the 43 individuals (males = 23, females = 20) sampled in this area, five were pregnant females (25% of the total females sampled) (Araújo 1998; Charvet-Almeida et al. 2005). The copulation period was identified during the rainy season (December–May), and pregnancy lasted six months (Charvet-Almeida et al. 2005). Between 2012–2015, 14 Schroeder’s Freshwater Stingrays were sampled during a study (Duncan et al. 2021). Individuals had an average body size of 17.7 cm DW (± 5.9 SD) with a range of 12.5–27.5 cm DW (Duncan et al. 2021). The size-at-birth for the species is 12–14 cm DW (Araújo et al. submitted a). Therefore, neonates and YOYs were present in this study.
Historical evidence from the mid-1990’s has shown that Wallace’s Freshwater Stingrays use this area for reproduction. From the 153 individuals (males = 83, females = 70) in this area, 35 were pregnant females (50% of the total females sampled) (Araújo 1998; Charvet-Almeida et al. 2005). Within this region, the reproductive cycle is seasonal and regulated by the flooding pulse of the Rio Negro, with copulation during the rainy season and a pregnancy with a period of three month (Araújo 1998; Charvet-Almeida et al. 2005). Several expeditions between 2006–2018 collected individuals in various life-stages, including neonates and YOYs, pregnant females, and sexually active males (de Oliveira et al. 2016; Duncan et al. 2016; Marcon et al. 2021; Siqueira‐Souza et al. 2024). During 10 field expeditions between December 2006 and October 2010, 357 Wallace’s Freshwater Stingrays were caught: 28 neonates, 10 YOYs, 76 subadults, and 243 adults (129 adult males and 114 adult females, of which 29 were pregnant; de Oliveira et al. 2016). Between 2012–2015, 327 Wallace’s Freshwater Stingrays (159 females, 168 males) were sampled, with ~30% of the individuals being neonates and YOYs (Duncan et al. 2016). In Lagoa Cuba, within this area, neonates and young individuals are frequently found on the flat and sandy banks along the small watercourses (Duncan et al. 2016). Parturition occurs during the low tide and early flooding of rivers and, during this period, more than 40% of adult females are pregnant (Duncan et al. 2016). Mating occurs during the low water period (September–October) and the young are born at the beginning of the rising water period (January–February). Size-at-birth varies between 6–10 cm DW and they reach maturity at 16–19 cm DW (Last et al. 2016). From January to May 2017, adult male Wallace’s Freshwater Stingrays (n = 41) were captured during the flooding season using a fishing net. A total of nine sexually active males were sampled (Marcon et al. 2021). Between November 2017 and May 2018 through scientific fishing using net, adult female Wallace’s Freshwater Stingrays were studied in different reproductive periods: resting stage (n = 15), gestation (i.e., foetuses measured 7–10 cm DW, indicating that they were nearly full‐term; n = 46), postpartum (i.e., without embryos and with a dilated uterus, indicating recent birth n = 9) (Siqueira‐Souza et al. 2024).
CRITERION D
SUB-CRITERION D2 – DIVERSITY
Negro Mariuá Archipelago sustains a high diversity of Qualifying Species (three species). This equals the regional diversity threshold (three species) for the South American Inland Waters region. The regular presence of Qualifying Species has been documented through various studies (Araújo 1998, 2011; de Oliveira et al. 2016, 2021; Duncan et al. 2016, 2021; Marcon et al. 2021; Siqueira‐Souza et al. 2024).
Download factsheet
SUBMIT A REQUEST
ISRA SPATIAL LAYER REQUEST
To make a request to download the ISRA Layer in either a GIS compatible Shapefile (.shp) or Google Earth compatible Keyhole Markup Language Zipped file (.kmz) please complete the following form. We will review your request and send the download details to you. We will endeavor to send you the requested files as soon as we can. However, please note that this is not an automated process, and before requests are responded to, they undergo internal review and authorization. As such, requests normally take 5–10 working days to process.
Should you have questions about the data or process, please do not hesitate to contact us.