ISRA FACTSHEETS
ISRA FACTSHEETS
SOUTH AMERICAN ATLANTIC REGION
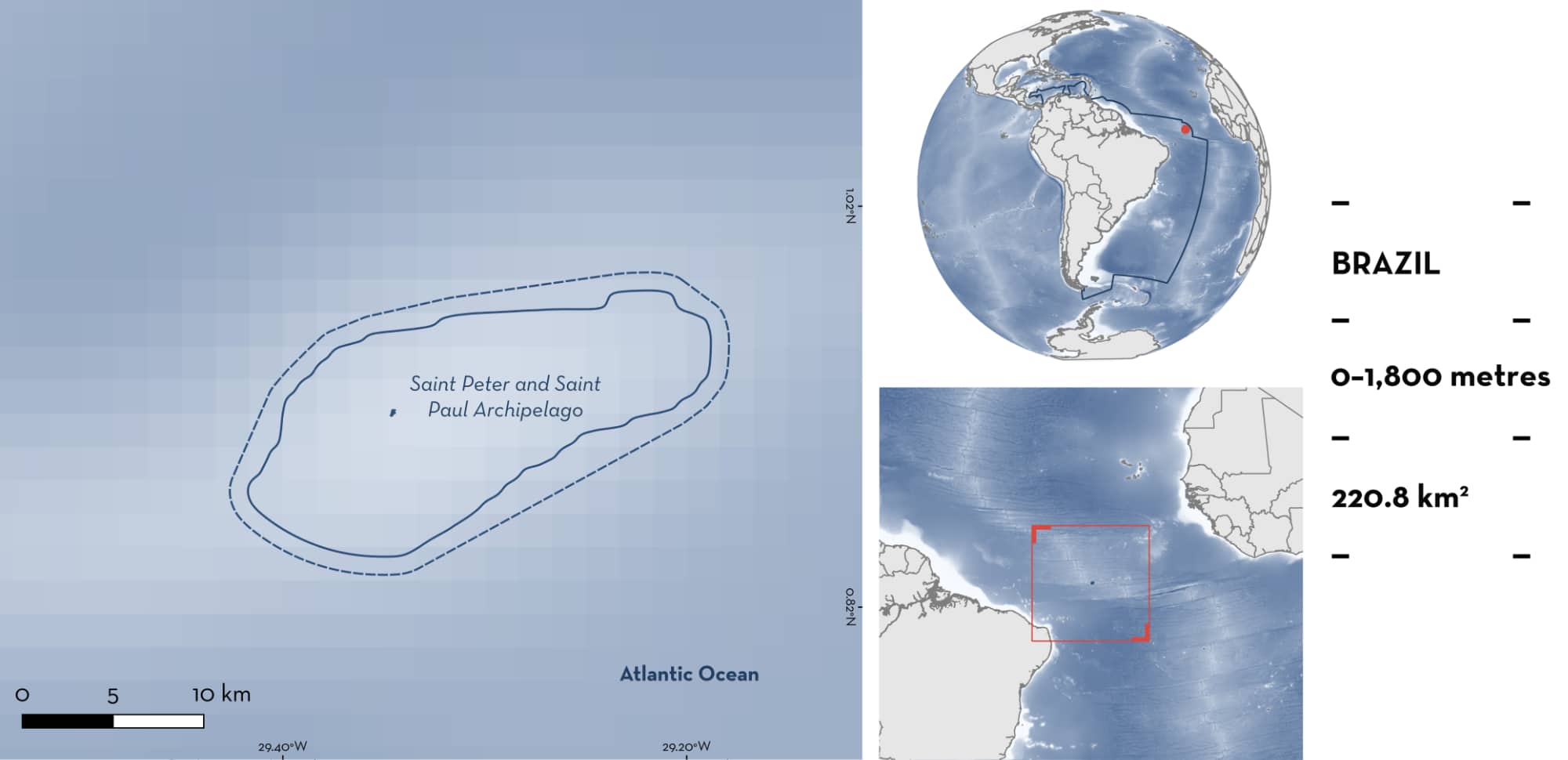
Saint Peter and Saint Paul Archipelago
Summary
Saint Peter and Saint Paul Archipelago is located in Brazil. It is a small group of rocky islets situated in the equatorial part of the Mid-Atlantic Ridge. From January to June, due to the oceanographic conditions, the water is enriched with zooplankton, fish eggs, and fish larvae. Around the islands, steep drop-offs and walls with rocky slopes and canyons are present. The area overlaps with the Natural Monument of Saint Peter and Saint Paul’s Archipelago and the Atlantic Equatorial Fracture Zone and high productivity system Ecologically or Biologically Significant Marine Area. Within this area there are: threatened species (e.g., Silky Shark Carcharhinus falciformis); reproductive areas (e.g., Sicklefin Devil Ray Mobula tarapacana); and feeding areas (Cookie-cutter Shark Isistius brasiliensis).
Download factsheet
Saint Peter and Saint Paul Archipelago
DESCRIPTION OF HABITAT
Saint Peter and Saint Paul Archipelago is located in Brazil. It is a small group of rocky islets situated along the equatorial part of the Mid-Atlantic Ridge, ~1,000 km away from Brazil and 1,800 km from Guinea Bissau. The archipelago is under the direct influence of the South Equatorial Current, which flows superficially in the east-west direction, and of the Equatorial Undercurrent, which flows in the opposite direction (west-east), between 60–100 m depth (Stramma & England 1999). The interaction of these currents with the local topography causes turbulent processes typically observed in seamounts, such as vortexes, stream velocity reductions, thermohaline structure disturbances, and local resurgence mechanisms that result in scattered enrichment of surface waters (Araujo & Cintra 2009). Due to these oceanographic conditions from January to June, the water is enriched with zooplankton, fish eggs, fish larvae, and invertebrates (Macedo-Soares et al. 2012). The reefs extend down to 100 m depth (Edwards & Lubbock 1983).
The shallow marine habitats comprise rocky shores, tide pools, and a small bay extending from 0 to 35 m depth, covered by the zoanthid Palythoa caribaeorum and algae of the genera Bryopsis, Caulerpa, and Dictyota. Surrounding the islands, steep drop-offs and walls with rocky slopes and canyons are present, descending to ~ 100 m, followed by another wall dropping between 130 and 140 m depth. The deeper regions encompass a large submarine mountain featuring extensive rocky reef habitats within the mesophotic and disphotic zones (Pinheiro et al. 2020).
The seabed between 150–600 m depth consists of large outcrops, boulder fields, and soft substrates dominated by coarse sediments, with finer grains observed at specific sites. Outcrops and boulders are extensively covered by filter-feeding benthic organisms such as sponges, cnidarians, echinoderms, and polychaetes, while the soft substrates are heavily colonised by brittle stars and other invertebrates (Pinheiro et al. 2020).
The area overlaps with the Natural Monument of Saint Peter and Saint Paul’s Archipelago (UNEP-WCMC & IUCN 2024) and the Atlantic Equatorial Fracture Zone and high productivity system Ecologically or Biologically Significant Marine Area (EBSA; CBD 2025).
This Important Shark and Ray Area is pelagic and delineated from surface waters (0 m) to a depth of 1,800 m based on the bathymetry of the area.
CRITERION A
VULNERABILITY
Three Qualifying Species considered threatened with extinction according to the IUCN Red List of Threatened Species regularly occur in the area. These are the Endangered Sicklefin Devil Ray (Marshall et al. 2022a) and Bentfin Devil Ray (Marshall et al. 2022b); and the Vulnerable Silky Shark (Rigby et al. 2021).
CRITERION C
SUB-CRITERION C1 – REPRODUCTIVE AREAS
Saint Peter and Saint Paul Archipelago is an important reproductive area for two shark and two ray species.
Between October 2010 and June 2015, 101 longline deployments were conducted during a fishery research survey across 60 days over 16 expeditions (Oliveira 2017). Of these, 65% occurred in the eastern part of the area, and 35% in the west. Each longline consisted of a 6 mm nylon monofilament mainline, 300–500 m in length, with 11–27 branch lines fitted with circle hooks (size 16 or 17) at their ends. Two fishing operations were conducted nightly: the first shortly after sunset, retrieved near midnight, and the second immediately after, retrieved before sunrise. The average soak time was four hours and 49 minutes (±1 hour and 38 minutes). In the 101 deployments, 129 sharks and rays were captured.
Silky Sharks comprised the majority of the captures (66.9%, n = 81), of which 76 individuals were measured and dissected (70–295 cm total length [TL]) (Oliveira 2017). Of these individuals, 24 were neonates/young-of-the-year (YOY) measuring <120 cm TL (Oliveira 2017). The smallest individual was a neonate with an umbilical scar measuring 70 cm TL. The size-at-birth of this species is 56–87 cm TL (Ebert et al. 2021). Historical data collected between September 1998 and April 2004 support the longstanding reproductive importance of this area (Hazin et al. 2007). From 96 Silky Sharks caught in this area, eight were classified as neonates/YOY (<120 cm) and ten females were pregnant. Embryo size from January–February varied widely (11–77.5 cm TL), suggesting no marked seasonal gestation cycle (Hazin et al. 2007).
For Cookie-cutter Shark, commercial fishing operations occurred at a maximum depth of 50 m during the day using handlines and trolling baited with flying fish and artificial bait, respectively (Santos et al. 2024). The survey spanned from March to September 2018, with effort distributed over four fishing cruises totalling 70 days at sea. A total of 200 individuals of five species of large pelagics (e.g., Wahoo Acanthocybium solandri and Yellowfin Tuna Thunnus albacares) were collected with bite marks from Cookie-cutter Sharks. The estimated size of sharks was calculated from the diameter of wounds using a linear regression of the shark’s mouth width to the TL of the sharks (Cadenat & Blache 1981; Muñoz-Chápuli et al. 1988). The length at 50% maturity (L50) for males was 34.6 cm TL and 41 cm TL for females (Compagno 1984). A total of 112 recent bites were used to estimate shark size. Cookie-cutter Sharks had an estimated length distribution from 6.2–23.3 cm TL and a mean estimated shark size of 13.8 ± 3.2 cm TL (Santos et al. 2024), indicating those were neonate/YOY. Size-at-birth for this species is 14–15 cm TL (Ebert et al. 2021). No other research focusing on Cookie-cutter Shark has been done in this area, but observations from researchers and fishers support their regular occurrence. Two juvenile Cookie-cutter Sharks were captured as incidental catch in the area, the first in August 2016 (~20 cm TL) and the second in August 2022 (23.5 cm TL) (Pinheiro et al. 2020; S Mendonça unpubl. data 2022). Size-at-maturity ranges between ~31–44 cm TL (Ebert et al. 2021). Between 2005–2015, during scientific cruises, at least 50% of big pelagic fishes captured had bite marks from Cookie-cutter Sharks (B Macena pers. obs. 2005–2015).
For Sicklefin Devil Ray, between December 2008 and June 2016, 38 expeditions of 15 days each (total = 570 days), were undertaken. The majority of expeditions (76%) occurred in the first half of the year – the period of greatest abundance of Sicklefin Devil Ray in the area (Mendonça et al. 2018; Mendonça et al. 2020). Sicklefin Devil Ray observations were performed mainly during the daytime and were restricted to one location on the western side of the islets. Observations were conducted from the deck of a fishing vessel or from an inflatable boat. Whenever the rays approached the survey point, free diving was conducted for underwater observation. From December 2008 to November 2010, sightings of 507 Sicklefin Devil Rays were recorded. Between December 2010 and June 2016, 320 sightings were recorded (Mendonça et al. 2018; Mendonça et al. 2020). Sex could be identified 361 times (43.6%), with females being more frequent (n = 215; 59.6%) than males (n = 146; 40.4%). The estimated disc width (DW) was recorded for 179 rays (22%) and ranged from 200–320 cm DW, with a mean ± SD of 260 ± 19 cm for both sexes pooled (Mendonça et al. 2020). Size at maturity is 270–280 cm DW for females and 198–250 cm DW for males (Notarbartolo-di-Sciara 1988; White et al. 2006; Rambahiniarison et al. 2018). Groups of 2–24 individuals were reported from 187 observations during the study (mean ± SD = 4.3 ± 3.6). The highest frequency of groups (n = 81) was observed in April and May. Solitary individuals were observed on 161 occasions (Mendonça et al. 2020). Evidence of mating was observed in both males (n = 7) and females (n = 6). In males, abrasions and swollenness/deformities were observed in the claspers while the females had scars in one or both pectoral fins. These potential mating scars were observed in April (2010, 2014, and 2015) and May (2014 and 2015), the same months when the largest groups were also seen (Mendonça et al. 2020). In addition to the mating scars, courtship behaviours were observed 14 times, mainly chasing trains, where sometimes the female was chased by one, two, or more males with the rays swimming in circles and overlapping with each other. Males made pelvic movements up and down, during courtship, in circles, apparently preparing for copulation although observations of chasing trains did not last more than a minute, with the rays moving away quickly (Mendonça et al. 2020). In some instances, during March and June, feeding behaviour (i.e., cephalic fins fully extended, and mouth opened) by single individuals or by groups of rays was observed concomitantly with other mobulid species that also feed on small pelagic organisms (Mendonça et al. 2018).
For Bentfin Devil Ray, one pregnant animal (180 cm DW) was caught by a commercial fishery boat in a small longline in March 2010. The left uterus contained one embryo at the initial stage of development (Mendonça et al. 2012). In May 2015, mating behaviour was recorded by drone during a scientific expedition, including both the ‘close following’ and attempted ‘pre-copulatory biting’ phase (McCallister et al. 2020). Additionally, aggregations of 3-15 animals, swollen claspers, and courtship events were observed between March and May in 2010, 2014, and 2015 (S Mendonça unpubl. data 2024).
CRITERION C
SUB-CRITERION C2 – FEEDING AREAS
Saint Peter and Saint Paul Archipelago is an important feeding area for one shark species.
Commercial fishing operations occurred at a maximum depth of 50 m during the day using handlines and trolls baited with flying fish and artificial bait, respectively. The survey spanned from March to September 2018, with effort distributed over four fishing cruises totalling 70 days at sea. A total of 200 individuals of five species were collected with bite marks by the Cookie-cutter Shark, comprising 100 Wahoo, 75 Yellowfin Tuna, 12 Common Dolphinfish Coryphaena hippurus, 11 Skipjack Tuna Katsuwonus pelamis, and two Rainbow Runner Elagatis bipinnulata. Many of the marks observed were recent bites that were fresh with open wounds (Yellowfin Tuna = 64.3%, Common Dolphinfish = 50%, Skipjack Tuna = 63.7, and Rainbow Runner = 50%), except for Wahoo for which most of the marks were already in the healing process (60.5%). A few scars were found only for two species, Wahoo (2.6%) and Yellowfin Tuna (19.7%). No other research focusing on Cookie-cutter Sharks has been done in this area, but observations from researchers and fishers support they are regularly feeding. Two juvenile Cookie-cutter Sharks were captured as incidental catch in the area, the first in August 2016 (~20 cm TL), attached to a Yellowfin Tuna, and the second in August 2022 (23.5 cm TL) (Pinheiro et al. 2020; S Mendonça unpubl. data 2022). Between 2005–2015, during scientific cruises, at least 50% of big pelagic fishes captured had bite marks from Cookie-cutter Sharks (B Macena pers. obs. 2005–2015). All analysed species presented at least one recent bite, indicating that Cookie-cutter Sharks actively feed on these species in the region, independent of fishing activities (Santos et al. 2024). The two species with the highest incidence of bites are predominantly oceanic, exhibiting epipelagic habits and occasionally venturing into mesopelagic layers. These species display migratory behaviour and primarily utilise the area for feeding (Viana et al. 2015). Unique geological features, combined with latitude, climatic conditions, marine currents, and biogeographic characteristics, make this area particularly attractive to large pelagic species, reinforcing its importance as a feeding ground for Cookie-cutter Sharks (Cruz et al. 2022).
Download factsheet
SUBMIT A REQUEST
ISRA SPATIAL LAYER REQUEST
To make a request to download the ISRA Layer in either a GIS compatible Shapefile (.shp) or Google Earth compatible Keyhole Markup Language Zipped file (.kmz) please complete the following form. We will review your request and send the download details to you. We will endeavor to send you the requested files as soon as we can. However, please note that this is not an automated process, and before requests are responded to, they undergo internal review and authorization. As such, requests normally take 5–10 working days to process.
Should you have questions about the data or process, please do not hesitate to contact us.